Abstract
Background. There are different opinions about the effect of functional ovarian cysts on the duration of controlled ovarian hyperstimulation, the dose of gonadotropins, the number and quality of collected oocytes and produced embryos, and the frequency of pregnancy.
Aim. To analyze in vitro fertilization (IVF) programs in women with anovulatory infertility and ovarian retention.
Materials and methods. A prospective study included 71 women aged 18–44 years. The main group (I) included patients (n = 38) with anovulatory infertility and functional ovarian cysts (FOC) diagnosed by ultrasound before enrollment in the IFV program. Patients of this group underwent ultrasound-guided transvaginal puncture of ovari- an cyst followed by cytology. The comparison group (II) (n = 33) encompassed patients with anovulatory infertility without FOC, who went through the IVF program. The control group (III) included apparently healthy individuals (n = 15).
The study algorithm included collection of clinical and anamnestic data of the patients, data of laboratory and instrumental studies, parameters of a stimulated IVF cycle, characteristics of oogenesis and early embryogenesis, and assessment of IVF program effectiveness.
Conclusion. It was established that in FOC and anovulatory infertility, the number of collected oocytes was small- er; however, the number of the best quality embryos and the frequency of pregnancy did not differ.
Aim. To analyze in vitro fertilization (IVF) programs in women with anovulatory infertility and ovarian retention.
Materials and methods. A prospective study included 71 women aged 18–44 years. The main group (I) included patients (n = 38) with anovulatory infertility and functional ovarian cysts (FOC) diagnosed by ultrasound before enrollment in the IFV program. Patients of this group underwent ultrasound-guided transvaginal puncture of ovari- an cyst followed by cytology. The comparison group (II) (n = 33) encompassed patients with anovulatory infertility without FOC, who went through the IVF program. The control group (III) included apparently healthy individuals (n = 15).
The study algorithm included collection of clinical and anamnestic data of the patients, data of laboratory and instrumental studies, parameters of a stimulated IVF cycle, characteristics of oogenesis and early embryogenesis, and assessment of IVF program effectiveness.
Conclusion. It was established that in FOC and anovulatory infertility, the number of collected oocytes was small- er; however, the number of the best quality embryos and the frequency of pregnancy did not differ.
Conflict of interest. The authors declare the absence of obvious and potential conflicts of interest related to the
publication of this article.
Source of financing. The authors state that they received no funding for the study.
Conformity with the principles of ethics. All patients signed an informed consent to participate in the study. The study was approved by the Ethics Committee at Siberian State Medical University (Protocol No. 9455 of 27.04.2023).
publication of this article.
Source of financing. The authors state that they received no funding for the study.
Conformity with the principles of ethics. All patients signed an informed consent to participate in the study. The study was approved by the Ethics Committee at Siberian State Medical University (Protocol No. 9455 of 27.04.2023).
Introduction
The problems of diagnosis and treatment of infertility are some of the main clinical and socially sensitive problems in modern medicine, which is overcome infertility [2]. According to the register of the Russian Association of Human Reproduction, more than 36,000 children were born following IVF in Russia in 2019 and more than 34,000 children were born in 2020 [3]. The indication for IVF is the ineffectiveness of infertility treatment by other methods for 12 months for women younger than 35 years and for 6 months for women older than 35 years [4].
According to the literature, every fourth patient with infertility has ovarian masses of various genesis and 80% of them are follicular ovarian cysts. The majority of retention masses are functional ovarian cysts (FOC): follicular cysts and corpus luteum cysts [5–9]. Over the last decade, the prevalence of FOC has increased from 6–12 to 25% [9–13]. In 60% of cases, they occur in patients of the reproductive age [14, 15]. Infertility in patients with FOC reaches 41% [16, 17].
The pelvic ultrasound examination is the starting point for planning ART and its purpose is to assess the endometrium, detect uterine cavity anomalies, count the number of antral follicles, and detect ovarian pathology, in particular ovarian cysts [4].Management strategy for patients with FOC in IVF programs remains controversial. B. Kumbak et al. (2009), E.B. Rudakova et al. (2014) believe that FOC do not affect the effectiveness of IVF programs [18, 19]. H.S. Qublan et al. (2006), R.D. Firouzabadiet al. (2010), and R. Levi et al. (2003) suggest that FOCs detected before the start of stimulation in IVF programs adversely affect its outcome as they require higher doses of gonadotropins, are associated with a lower ovarian response, and reduce the pregnancy rate [20–22].
There are different approaches to the management of patients with FOC in ART programs: surgical (transvaginal puncture of the cyst with a subsequent cytological examination) and conservative (administration of gonadotropin-releasing hormone (GnRH) agonists and antagonists for 3–7 days) methods, as well as a wait-and-see approach [18–27]. Despite a significant number of studies, the impact of FOC on the course and outcome of IVF protocols, namely on the features of controlled ovarian superovulation, the number and quality of oocytes, as well as the parameters of oogenesis and early embryogenesis, requires further research.
According to the literature, every fourth patient with infertility has ovarian masses of various genesis and 80% of them are follicular ovarian cysts. The majority of retention masses are functional ovarian cysts (FOC): follicular cysts and corpus luteum cysts [5–9]. Over the last decade, the prevalence of FOC has increased from 6–12 to 25% [9–13]. In 60% of cases, they occur in patients of the reproductive age [14, 15]. Infertility in patients with FOC reaches 41% [16, 17].
The pelvic ultrasound examination is the starting point for planning ART and its purpose is to assess the endometrium, detect uterine cavity anomalies, count the number of antral follicles, and detect ovarian pathology, in particular ovarian cysts [4].Management strategy for patients with FOC in IVF programs remains controversial. B. Kumbak et al. (2009), E.B. Rudakova et al. (2014) believe that FOC do not affect the effectiveness of IVF programs [18, 19]. H.S. Qublan et al. (2006), R.D. Firouzabadiet al. (2010), and R. Levi et al. (2003) suggest that FOCs detected before the start of stimulation in IVF programs adversely affect its outcome as they require higher doses of gonadotropins, are associated with a lower ovarian response, and reduce the pregnancy rate [20–22].
There are different approaches to the management of patients with FOC in ART programs: surgical (transvaginal puncture of the cyst with a subsequent cytological examination) and conservative (administration of gonadotropin-releasing hormone (GnRH) agonists and antagonists for 3–7 days) methods, as well as a wait-and-see approach [18–27]. Despite a significant number of studies, the impact of FOC on the course and outcome of IVF protocols, namely on the features of controlled ovarian superovulation, the number and quality of oocytes, as well as the parameters of oogenesis and early embryogenesis, requires further research.
The aim of the study was to analyze IVF programs in patients with anovulatory infertility and FOCs detected in the IVF program.
Materials and methods
We conducted a prospective study that included 71 women of reproductive age who applied to the Center for Assisted Reproductive Technologies of Siberian State Medical University. The main group (I) consisted of (n = 38) patients with anovulatory infertility and FOCs diagnosed by ultrasound immediately before enrollment in the IVF program. Patients in thisgroup underwent ultrasound-guided transvaginal cyst puncture followed by the cytological examination. The comparison group (II) (n = 33) included patients with anovulatory infertility without FOC who went through IVF. The control group (III) included healthy patients (n = 15).
Inclusion criteria:
1) age 18–44 years;
2) anti-Müllerian hormone (AMH) level in the
blood of women 1.2–3.5 ng / ml;
3) informed consent to participate in this study;
4) body mass index (BMI) 18.5–30.0.
Exclusion criteria:
1) the age under 18 and over 45 years;
2) hyperprolactinemia;
3) moderate and severe forms of genital
endometriosis (ASRM ≥ III, 1996);
4) hypothyroidism;
5) uterine myoma requiring surgical intervention; 6) true ovarian tumors;
7) contraindications to IVF according to the
Order of the Ministry of Healthcare of the Russian Federation No. 803n of 31.07.2020 “On the Procedure for the Use of Assisted Reproductive Technologies, Contraindications, and Restrictions on Their Use” (hereinafter – Order No. 803n);
8) the woman’s refusal to participate in the study.
The main group included patients with unilateral FOC persisting for no more than 3 months and being 25–60 mm in diameter according to the ultrasound examination. In case of FOC detection, patients were tested for CA-125 and HE-4. If the concentration of these markers increased above the reference values, the patient was excluded from the study.
The research algorithm included an analysis of clinical characteristics and medical history of the patients, laboratory tests and clinical investigation data, stimulated cycle parameters, oogenesis and early embryogenesis parameters, as well as an assessmentof the effectiveness of the IVF treatment. All patients were examined in accordance with the Order No. 803n. All patients underwent ovulation stimulation according to the protocol with GnRH antagonists. Statistical data was processed using the SPSS 23 program. The Levene’s test was used to determine the homogeneity of dispersions. The Kolmogorov – Smirnov test with the Lilliefors correction, the Shapiro – Wilk test, and visual assessment of histograms were used to assess the normal distribution of variables. The Mann – Whitney test was used to compare quantitative data from two independent groups, and the Kruskal – Wallis test was used to compare data from more than two independent groups, followed by an analysis of a posteriori comparisons by the Dunn’s test (Dunn, 1964) and the Conover– Iman test (Conover, Iman, 1979). When comparing qualitative variables, contingency tables with the Pearson’s χ2 test were used. The data were presented as the median and the interquartile range (Me (Q25–Q75)), or the mean and the standard error (M ± SD). The critical significance level p for all statistical analysis procedures was 0.05.
Inclusion criteria:
1) age 18–44 years;
2) anti-Müllerian hormone (AMH) level in the
blood of women 1.2–3.5 ng / ml;
3) informed consent to participate in this study;
4) body mass index (BMI) 18.5–30.0.
Exclusion criteria:
1) the age under 18 and over 45 years;
2) hyperprolactinemia;
3) moderate and severe forms of genital
endometriosis (ASRM ≥ III, 1996);
4) hypothyroidism;
5) uterine myoma requiring surgical intervention; 6) true ovarian tumors;
7) contraindications to IVF according to the
Order of the Ministry of Healthcare of the Russian Federation No. 803n of 31.07.2020 “On the Procedure for the Use of Assisted Reproductive Technologies, Contraindications, and Restrictions on Their Use” (hereinafter – Order No. 803n);
8) the woman’s refusal to participate in the study.
The main group included patients with unilateral FOC persisting for no more than 3 months and being 25–60 mm in diameter according to the ultrasound examination. In case of FOC detection, patients were tested for CA-125 and HE-4. If the concentration of these markers increased above the reference values, the patient was excluded from the study.
The research algorithm included an analysis of clinical characteristics and medical history of the patients, laboratory tests and clinical investigation data, stimulated cycle parameters, oogenesis and early embryogenesis parameters, as well as an assessmentof the effectiveness of the IVF treatment. All patients were examined in accordance with the Order No. 803n. All patients underwent ovulation stimulation according to the protocol with GnRH antagonists. Statistical data was processed using the SPSS 23 program. The Levene’s test was used to determine the homogeneity of dispersions. The Kolmogorov – Smirnov test with the Lilliefors correction, the Shapiro – Wilk test, and visual assessment of histograms were used to assess the normal distribution of variables. The Mann – Whitney test was used to compare quantitative data from two independent groups, and the Kruskal – Wallis test was used to compare data from more than two independent groups, followed by an analysis of a posteriori comparisons by the Dunn’s test (Dunn, 1964) and the Conover– Iman test (Conover, Iman, 1979). When comparing qualitative variables, contingency tables with the Pearson’s χ2 test were used. The data were presented as the median and the interquartile range (Me (Q25–Q75)), or the mean and the standard error (M ± SD). The critical significance level p for all statistical analysis procedures was 0.05.
Results
The mean age of all the examined individuals (n = 71) was 34.0 (30.0–39.0) years. The statistical analysis showed age homogeneity of the main group and the comparison group (p = 0.746). In the vast majority of cases, menarche in women of all groups occurred at the age of 12–15 years. The mean age of menarche in all the subjects (n = 71) was 13.5 (12.0–14.0) years. The mean duration of the menstrual cycle in all the subjects (n = 71) was 30.0 (27.0–37.0) days. Menstrual disorders were established in the medical history of 51 (71.8 %) patients. Oligomenorrhea occurred in 12 (16.9%) patients, while abnormal uterine bleeding (AUB) in the absence of chronic endometrial pathology was observed in 20 (28.1%) women. Dysmenorrhea was observed in 21 (29.5%) patients. The statistical analysis of groups I and II showed their homogeneity in all the studied menstrual cycle parameters.
The mean duration of infertility was 6.0 ± 0.3 years (p = 0.929). Combined infertility (combination of both male and female factors) occurred in 19 (26.7 %) patients. Combined female infertility was diagnosed in 25 (35.2 %) patients. Tubal factor and endometriosis were detected in 18 (25.4 %) and 12 (12.1 %) patients, respectively. The statistical analysis of homogeneity in groups I and II did not reveal any differences.
The health status of all patients was assessed as satisfactory. At the time of enrollment in the ART program, 50.5% of women had extragenital pathology that was in a stable remission or compensated. Compensated pathology of the thyroid gland in 35.2% of the patients and pathology of the gastrointestinal tract in remission in 16.9% of patients of all groups were the most common.
Hormonal profile (AMH, follicle stimulating hormone (FSH), luteinizing hormone (LH)) and ultrasound data on the antral follicle count (AFC) were used to assess the ovarian reserve of patients in all groups (Table 1).
The mean duration of infertility was 6.0 ± 0.3 years (p = 0.929). Combined infertility (combination of both male and female factors) occurred in 19 (26.7 %) patients. Combined female infertility was diagnosed in 25 (35.2 %) patients. Tubal factor and endometriosis were detected in 18 (25.4 %) and 12 (12.1 %) patients, respectively. The statistical analysis of homogeneity in groups I and II did not reveal any differences.
The health status of all patients was assessed as satisfactory. At the time of enrollment in the ART program, 50.5% of women had extragenital pathology that was in a stable remission or compensated. Compensated pathology of the thyroid gland in 35.2% of the patients and pathology of the gastrointestinal tract in remission in 16.9% of patients of all groups were the most common.
Hormonal profile (AMH, follicle stimulating hormone (FSH), luteinizing hormone (LH)) and ultrasound data on the antral follicle count (AFC) were used to assess the ovarian reserve of patients in all groups (Table 1).

Thus, the analysis of ovarian reserve data shows the homogeneity of groups I and II. The homogeneity of the groups was achieved by fulfilling the inclusion criteria, namely limiting the AMH level to 1.2–3.5 ng / ml and BMI for participation in the study. Thus, all the studied groups of patients had the expected normal response to ovulation stimulation, which makes it possible to obtain true data on the influence of FOC on the effectiveness of ovarian stimulation. Ovulation stimulation in all patients who participated in the study was performed according to a protocol using GnRH antagonists.
GnRH antagonists were added on day 6 of gonadotropin stimulation. The study of induced cycle parameters in the main group and the comparison group did not reveal any statistically significant differences in either the starting dose or the duration of stimulation (Table 2).
GnRH antagonists were added on day 6 of gonadotropin stimulation. The study of induced cycle parameters in the main group and the comparison group did not reveal any statistically significant differences in either the starting dose or the duration of stimulation (Table 2).

The effectiveness of the IVF program is expressed
not only by the response to ovulation stimulation, but also by the quantity and quality of oocytes and embryos obtained. Based on the data obtained, we analyzed the parameters of oogenesis and early embryogenesis. The average number of punctured follicles in group I and II (n = 38 and n = 33, respectively) was 10.2 (from 7 to 13). The number of punctured follicles was significantly higher in the comparison group with anovulatory infertility and absence of FOC than in the patients of the main group (p = 0.006). A significant increase in the number of obtained oocytes in the group of patients without FOC was also observed (p = 0.002). However, the proportion of mature oocytes was higher in the group with FOC (p = 0.014), which ultimately resulted in an equal number of best quality embryos in both groups (p = 0.097) (Table 3).
not only by the response to ovulation stimulation, but also by the quantity and quality of oocytes and embryos obtained. Based on the data obtained, we analyzed the parameters of oogenesis and early embryogenesis. The average number of punctured follicles in group I and II (n = 38 and n = 33, respectively) was 10.2 (from 7 to 13). The number of punctured follicles was significantly higher in the comparison group with anovulatory infertility and absence of FOC than in the patients of the main group (p = 0.006). A significant increase in the number of obtained oocytes in the group of patients without FOC was also observed (p = 0.002). However, the proportion of mature oocytes was higher in the group with FOC (p = 0.014), which ultimately resulted in an equal number of best quality embryos in both groups (p = 0.097) (Table 3).

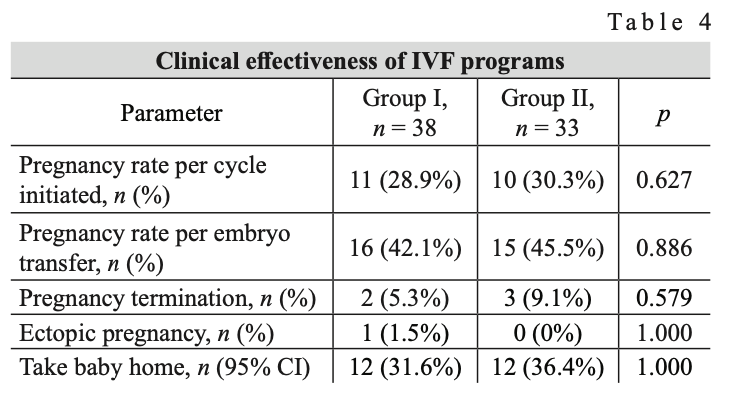
The effectiveness of IVF was analyzed (Table 4). The pregnancy rate per cycle initiated and per embryo transfer was 21/71 (29.5 %) and 31/71 (43.7 %) in group I and II, respectively. The Take baby home parameter, i.e. the delivery of a baby, was 24/71 (33.9 %). The Pearson’s χ2 test and contingency tables showed that implantation, pregnancy per cycle initiated (transfer), and live birth rates were similar regardless of the presence (absence) of FOC before entering the IVF treatment.
Conclusion
FOC may have a negative effect on the effectiveness of ovarian stimulation, which can manifest itself by a lower follicle count (p = 0.006) and a smaller number of oocytes produced (p = 0.002) in anovulatory infertility. This effect is probably mediated both by the mechanical effect of FOC on the ovary, which prevents follicle growth, and by an altered blood supply which prevents follicle development, resulting in a reduced response to stimulation and a smaller number of oocytes produced in the anovulatory infertility group with FOC compared to the group without FOC.
Presumably, FOC may have an active paracrine or endocrine effect resulting from the release of estradiol. Cysts associated with increased estradiol levels may cause a premature increase in LH and progesterone levels, which leads to a decrease in the oocyte quality and adversely affects the endometrium. The presumed mechanism of the negative effect of FOC on the number of oocytes during ovarian stimulation is consistent with the literature data [20, 23, 24]. However, the proportion of mature oocytes (MII) was higher in the group of patients with FOC compared to the group of patients with anovulatory infertility without FOC, which proves that there is no pronounced negative effect of follicular cysts and corpus luteum cysts on the quality and competence of oocytes.
The number of best quality embryos (category A and B blastocysts according to the Gardner blastocyst grading system aimed at assessing human embryos at the blastocyst stage proposed by Gardner, Schoolcraft (1999), based on the analysis of trophectoderms, inner cell mass, and blastocyst cavity size) also did not differ in the two groups (p = 0.097), which confirms the literature data on the absence of a possible negative effect of FOC on the effectiveness of the IVF program [18, 19]. Program effectiveness estimated as the number of pregnancies per cycle initiated and the number of pregnancies per transfer was also similar in both groups (p = 0.89 and p = 0.89, respectively). Thus, FOCs detected before patient entering the protocol do not have a pronounced adverse effect on the outcome of ART programs.
Presumably, FOC may have an active paracrine or endocrine effect resulting from the release of estradiol. Cysts associated with increased estradiol levels may cause a premature increase in LH and progesterone levels, which leads to a decrease in the oocyte quality and adversely affects the endometrium. The presumed mechanism of the negative effect of FOC on the number of oocytes during ovarian stimulation is consistent with the literature data [20, 23, 24]. However, the proportion of mature oocytes (MII) was higher in the group of patients with FOC compared to the group of patients with anovulatory infertility without FOC, which proves that there is no pronounced negative effect of follicular cysts and corpus luteum cysts on the quality and competence of oocytes.
The number of best quality embryos (category A and B blastocysts according to the Gardner blastocyst grading system aimed at assessing human embryos at the blastocyst stage proposed by Gardner, Schoolcraft (1999), based on the analysis of trophectoderms, inner cell mass, and blastocyst cavity size) also did not differ in the two groups (p = 0.097), which confirms the literature data on the absence of a possible negative effect of FOC on the effectiveness of the IVF program [18, 19]. Program effectiveness estimated as the number of pregnancies per cycle initiated and the number of pregnancies per transfer was also similar in both groups (p = 0.89 and p = 0.89, respectively). Thus, FOCs detected before patient entering the protocol do not have a pronounced adverse effect on the outcome of ART programs.
References
- WHO laboratory manual for the examination and processing of human seeds. 6th ed. World Health Organization, 2021. URL: https://www.who.int/publications/i/item/9789240030787 (in Russ.)
- Chambers G.M., Dyer S., Zegers-Hochschild F., de Mouzon J., Ishihara O., Banker M et al. International Committee for Monitoring Assisted Reproductive Technologies world re- port: assisted reproductive technology, 2014. Hum. Reprod. 2021;36(11):2921–2934. DOI: 10.1093/humrep/deab198.
- Russian Association of Human Reproduction. ART Register: report for 2020. URL: https://rahr.ru/registr_otchet.php (date of application: 03/06/2023) (in Russ.).
- Russian Society of Obstetricians and Gynecologists. Fe- male infertility: guidelines. 2021. URL: https://mz.mos- reg.ru/dokumenty/informaciya/klinicheskie-rekomendaci- i/02-08-2021-11-12-30-zhenskoe-besplodie (in Russ.)
- Fiorentino G., Cimadomo D., Innocenti F., Soscia D., Va- iarelli A., Ubaldi F.M. et al. Biomechanical forces and signals operating in the ovary during folliculogenesis and their dys- regulation: implications for fertility. Hum. Reprod. Update. 2023;29(1):1–23. DOI: 10.1093/humupd/dmac031.al.
- Dubrovina S.O., Berlim Yu.D., Gimbut V.S., Vovkochina M.A. Hormone therapy for functional ovarian cysts. Obstetrics and gynecology. 2020;(4):210–213 (in Russ.). DOI: 10.18565/ aig.2020.4.210-213.
- Shukurov F.I., Ayupova F.M. Role of adjuvant hormonal therapy in restoring reproductive function in women after en- dosurgical treatment of ovarian follicular cysts. Gynecology. 2021;23(1):68–72 (in Russ.). DOI: 10.26442/20795696.2021. 1.200441.
- Jaroslava D. Cytology of ovarian cysts. Cesk/ Patol. 2019;55(2):107–111.
- Podzolkova N.M., Osadchev V.B., Babkov K.V., Safono- va N.E. The differential diagnosis algorithm of ovarian tumors in reproductive patients: a prospective study. Gynecology. 2022;24(2):80–87 (in Russ.). DOI: 10.26442/20795696.2022. 2.201387.
10. Mateikovich E.A., Shevlyukova T.P., Chernova A.L. Benign tumors and tumor-like lesions of the ovaries: structure, diag- nostic methods, tactics of medical care. Medical science and education of the Urals. 2021;22(1):100–104 (in Russ.). DOI:
10.36361/1814-8999-2021-22-1-100-104.
11. Medzhidova K.K., Aliyeva Kh.G., Hasanova M.A., Aliye-
va D.Kh., Idrisov M.M., Magomedov R.G. Treatment of ovarian cysts. Russian Journal of Human Reproduction. 2014;(5):35–38 (in Russ.).
12. Matevosyan A.A. Modern aspects of the implementation of the IVF and PE program in women with benign ovarian tu- mors. Bulletin of Surgery of Armenia named after G.S. Tama- zyan. 2010;(2):38–42 (in Russ.).
13. Mobeen S., Apostol R. Ovarian cyst. In: Stat. Pearls [Internet]. Treasure Island (FL): Stat. Pearls Publishing, 2022. URL:https://www.ncbi.nlm.nih.gov/books/NBK560541
14. Sorokina I.V., Markovsky V.D., Borzenkova I.V., Kula- kova E.A., Miroshnichenko M.S., Pliten O.N., et al. Cystic ovarian formations in women: clinical and morphological features. Morphology. 2015;9(2):78–84 (in Russ.). DOI: 10.26641/1997-9665.2015.2.78-84.
15. Gasymova D.M., Rukhlyada N.N. Clinical and anamnes- tic features of patients with complications of benign tumors and ovarian tumors. Russian Bulletin of Obstetrician-Gy- necologist. 2017;17(4):72–77 (in Russ.). DOI: 10.17116/ro- sakush201717472-77.
16. Shapoval O.S., Reznichenko G.I. Features of the realization of reproductive function in women with benign tumor-like for- mations of the ovaries. A woman’s health. 2015;(2):104–107 (in Russ.).
17. Volchenok D.A., Tikhonovskaya O.A., Petrov I.A., Logvi- nov S.V., Mungalova A.D. The state of ovarian reserve in pa- tients with functional ovarian cysts. Journal of Siberian Med- ical Sciences. 2019;(1):18-27. Journal of Siberian Medical Sciences. 2019;(1):18–27. DOI: 10.31549/2542-1174-2019- 1-18-27.
18. Kumbak B., Kahraman S. Management of prestimulation ovarian cysts during assisted reproductive treatments: im- pact of aspiration on the outcome. Arch. Gynecol. Obstet. 2009;279(6):875–880. DOI: 10.1007/s00404-008-0837-7.
19. Rudakova E.B., Strizhova T.V., Trubnikova O.B., Zamak- hovskaya L.Yu. Ovarian cysts in IVF and PE programs in pro- tocols with AH-RG. Reproductive medicine. 2014;(34):11–13 (in Russ.).
20. Qublan H.S., Amarin Z., Tahat Y.A., Smadi A.Z., Kilani M. Ovarian cyst formation following GnRH agonist adminis- tration in IVF cycles: incidence and impact. Hum Reprod. 2006;21(3):640–644. DOI: 10.1093/humrep/dei371.
21. Firouzabadi R.D., Sekhavat L., Javedani M. The effect of ovar- ian cyst aspiration on IVF treatment with GnRH. Arch. Gyne- col. Obstet. 2010;281(3):545–549. DOI: 10.1007/s00404-009- 1195-9.
22. Levi R., Ozçakir H.T., Adakan S., Göker E.N., Tavmergen E. Effect of ovarian cysts detected on the beginning day of ovu- lation induction to the success rates in ART cycles. J. Obstet. Gynaecol. Res. 2003;29(4):257–261. DOI: 10.1046/j.1341- 8076.2003.00110.x.
23. Pereira N., Amrane S., Hobeika E., Lekovich J.P., Chung P.H., Rosenwaks Z. Cyst aspiration or GnRH antagonist administra- tion for ovarian cysts detected at the start of fresh in vitro fer- tilization cycles. Gynecol. Endocrinol. 2016;32(7):562–565.
- McDonnell R., Marjoribanks J., Hart R.J. Ovarian cyst aspi-
- ration prior to in vitro fertilization treatment for subfertility. Cochrane Database Syst. Rev. 2014;2014(12):CD005999. DOI: 10.1002/14651858.CD005999.pub2.
- Ji H., Su Y., Zhang M., Li X., Li X., Ding H. et al. Func- tional ovarian cysts in artificial frozen-thawed embryo trans- fer cycles with depot gonadotropin-releasing hormone ago- nist. Front. Endocrinol. (Lausanne). 2022;13:828993. DOI: 10.3389/fendo.2022.828993.
26. Kostrzewa M., Zając A., Wilczyński J.R., Stachowiak G. Retrospective analysis of transvaginal ultrasound-guid- ed aspiration of simple ovarian cysts. Adv. Clin. Exp. Med. 2019;28(11):1531–1535. DOI: 10.17219/acem/104549.
27. Farquhar C., Rombauts L., Kremer J.A., Lethaby A., Ayeleke R.O. Oral contraceptive pill, progestogen or oestrogen pre- treatment for ovarian stimulation protocols for women under- going assisted reproductive techniques. Cochrane Database Syst Rev. 2017;5(5):CD006109. DOI: 10.1002/14651858. CD006109.pub3.
Author's Contribution
Timofeeva O.S., Petrov I.A., Tikhonovskaya O.A., Logvinov S.V. – conception and design. Timofeeva O.S., Gaifulina J.F., Samoilo- va Yu.G., Petrova M.S., Yuriev S.Yu., Dmitrieva M.L., Zhdankina A.A., Gerasimov A.V., Mikheenko G.A. – data search and analysis. Timofeeva O.S. – drafting of the manuscript. Petrov I.A., Tikhonovskaya O.A., Logvinov S.V. – final approval of the manuscript for publication.


